What is quality management maturity?
Quality management maturity (QMM) refers to the degree an organization has embedded quality practices into its operational and strategic processes. But it's more than just compliance with standards or achieving certification; it's about adopting a comprehensive, organization-wide approach to continual quality improvement.
Organizations with a high level of quality maturity demonstrate a strong commitment to quality at all levels. They have robust quality management systems in place, clear quality objectives and regularly review performance against these targets. They also foster a culture of quality, encouraging employees to take ownership of quality outcomes.
Understanding your quality maturity provides a roadmap for improvement. By assessing and enhancing it, you can better operational efficiency, improve customer satisfaction, lower costs and gain a competitive edge.
Let's delve further into the concept of quality maturity, its implications and how it can be developed within an organization. We go into more detail about quality maturity in this article, but you can also find a link to our in-depth guide to quality maturity below, which significantly expands on the information you will find here.

Keen to learn more about how to turn an understanding of quality maturity into a competitive advantage for your organization? Download our quality maturity whitepaper today.
Download nowA basic quality maturity model
It’s helpful when assessing your organization’s quality management maturity to use a quality maturity model. A quality maturity model might look as follows:
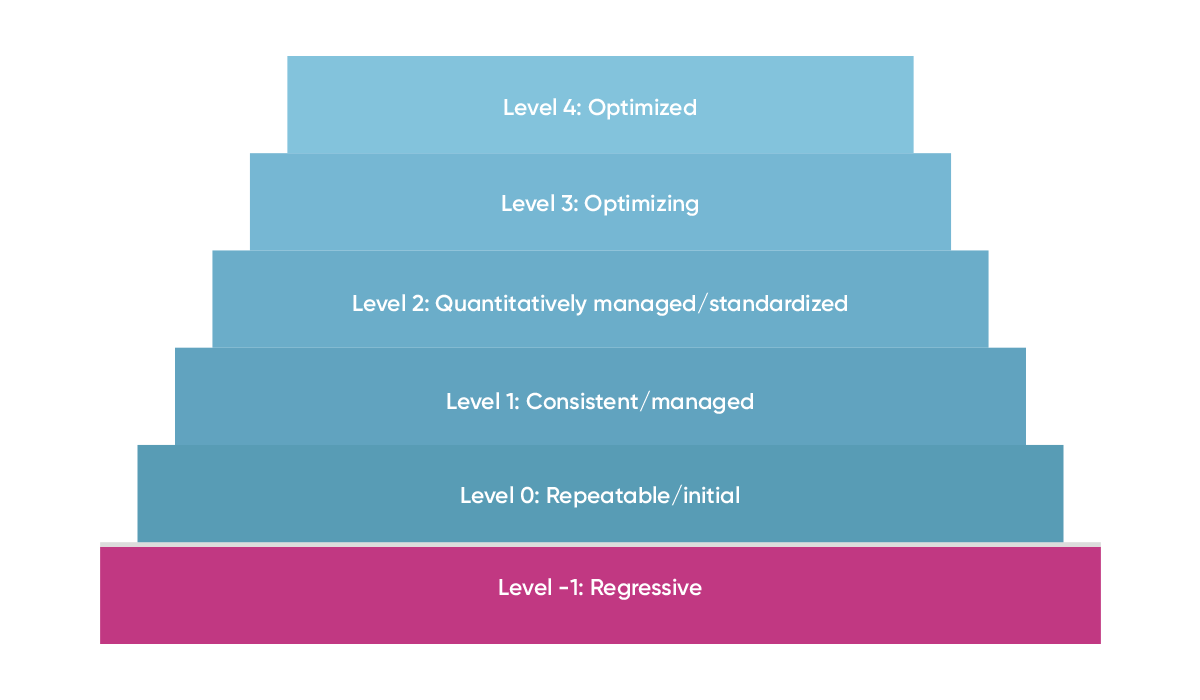
Below, we elaborate on what each of the levels of this maturity model mean in practice, so you can get a solid idea of where your organization might be on its quality management maturity journey.
Level -1: regressive: The organization lacks a systematic approach to quality management. In a pharmaceutical context, this could manifest as inconsistent product quality and high risk of product recalls.
Level 0: repeatable/initial: The organization has some repeatable processes in place. However, these efforts lack standardization.
Level 1: consistent/managed: Here, the organization has established consistent processes. There is regular compliance with quality standards and accountability for quality management has been assigned.
Level 2: quantitatively managed/standardized: The organization not only has consistent quality processes, but also uses data to manage performance and drive improvements.
Level 3: optimizing: In the optimizing stage, the organization continually seeks out ways to improve quality performance. For a pharmaceutical company, this might involve a proactive approach to quality enhancement.
Level 4: optimized: At this pinnacle stage, the organization has successfully embedded quality into its DNA. Processes are not only standardized and controlled but are optimized and continuously improved upon.
Achieving Level 4 doesn't mean the process is finished; there is always room for improvement. The journey of quality maturity is ongoing, with each stage serving as a foundation for the next. It's about striving for excellence, not just compliance.
Evaluating your organization's quality maturity level
To effectively utilize the quality management maturity model, it's vital to assess your organization's current standing and identify potential areas for improvement. You can do this by measuring your organization's efficacy in various operational areas such as product development, manufacturing, regulatory compliance, supply chain management and customer service against the defined maturity levels.
While assessing your organization's quality maturity level, it's crucial to consider both internal and external metrics. Internally, evaluate how well your quality management system is defined and understood by your employees. Examine your key performance indicators (KPIs) and other metrics used to track and improve quality.
Externally, gauge how your customers perceive your quality. Customer feedback can provide a wealth of insight into your quality performance. Regularly conduct customer satisfaction surveys and closely monitor product reviews and complaints.
Furthermore, it's essential to examine the quality of your suppliers. An efficient, reliable supply chain plays a vital role in maintaining high product quality. Regular audits of your suppliers' processes and products can help ensure they meet your quality standards.
By comprehensively assessing these areas, you will be able to clearly identify where your organization stands in terms of quality management maturity. This understanding will guide your strategic planning and drive continual quality improvement, propelling your organization forward on its journey toward quality excellence.
Quality management software: the key to unlocking quality management maturity
Quality management software can significantly enhance an organization's maturity in quality practices. At Ideagen, we offer powerful quality management solutions to help you achieve this goal. From compliance tracking to continuous improvement initiatives, these software solutions act as a central hub for all quality-related activities.
Quality management software is an instrumental tool in an organization's quality maturity journey, contributing to continuous improvement and a relentless pursuit of quality excellence.

Want to gain a better understanding of how Ideagen have the solutions to unlocking greater quality maturity levels? Unlock quality maturity with Ideagen today by learning more about our quality management software and request a demo with our team.
Request a demoTags:
